1. Food Surveillance System
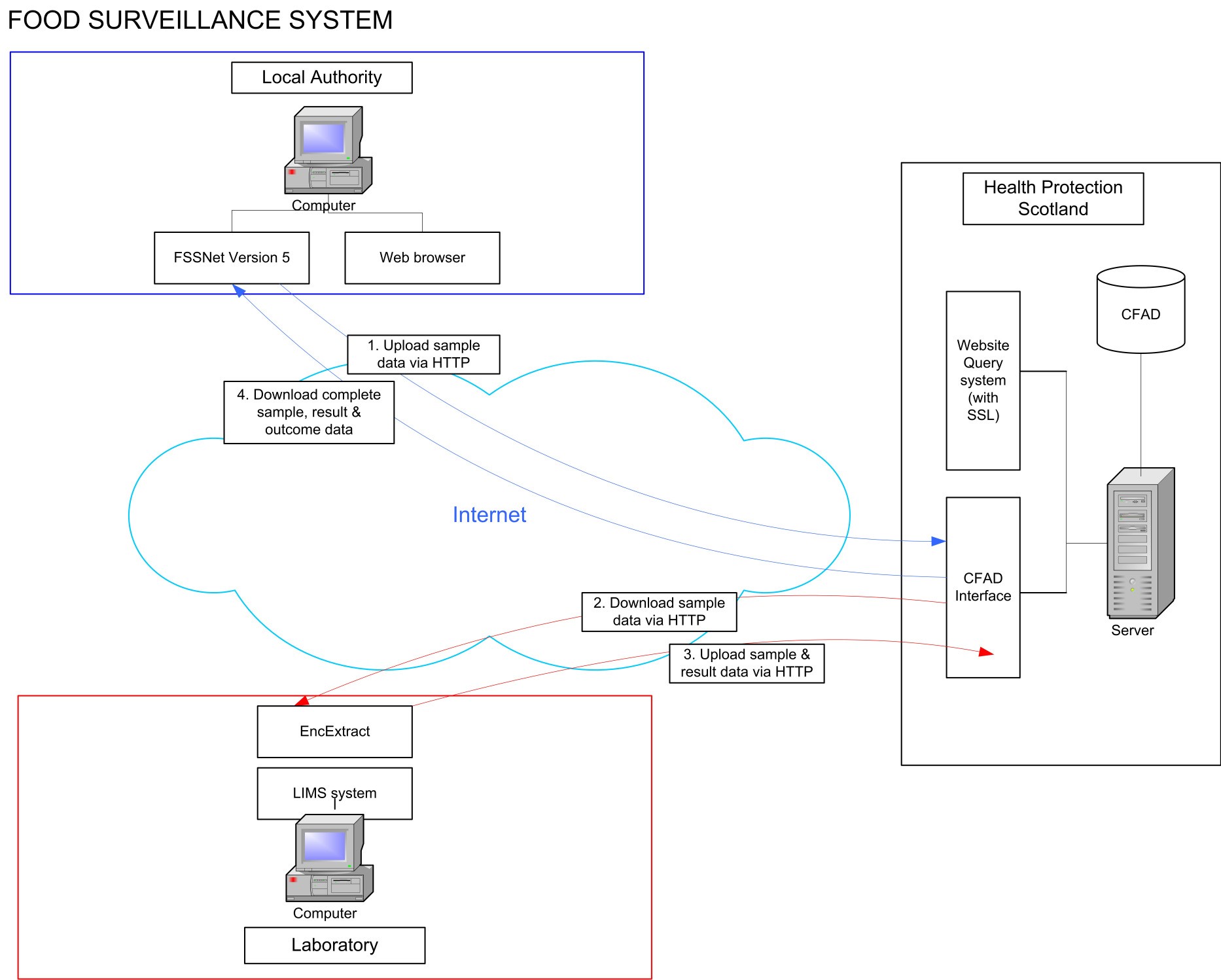
The Food Surveillance System collects food and animal feed sample information taken by competent authorities across the UK. The sample and results data is submitted to the central database by the laboratories.
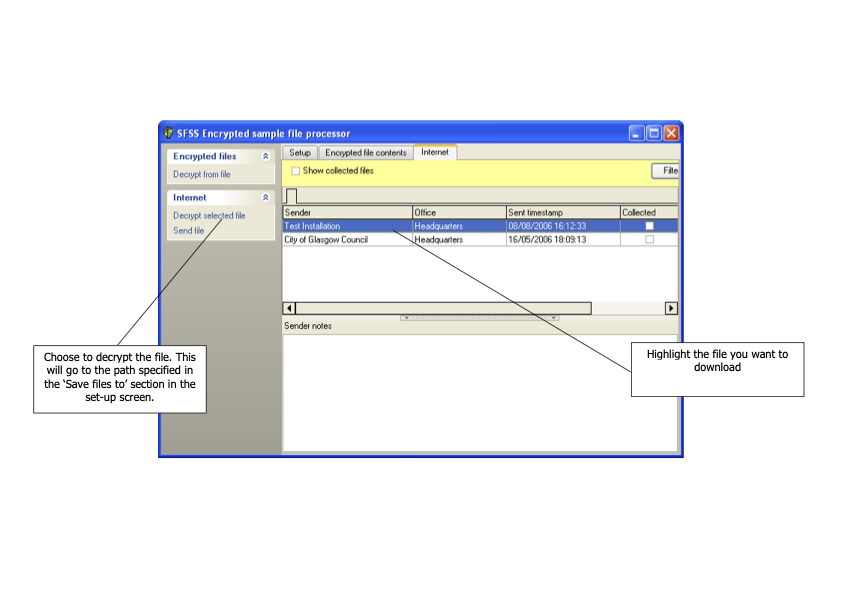
Food sampling officers enter details of samples taken and of the analysis required into the FSSNet software application. The data is exported and sent as an encrypted file to the relevant Public Analyst laboratory. The file may be sent on a floppy disk, a USB memory stick, as an e-mail attachment or via the internet. Each laboratory will agree the most efficient method with each of the participating clients. In some instances, it is convenient to allow the officers to enter the sample data into a computer running the FSS software which is located on the laboratory premises. A separate procedure is also available for the data file to be transferred between the Competent Authorities client and the laboratory by way of the FSS website.
The laboratory registers the samples into their own Laboratory Information Management System (LIMS) using the FSS data file for much of the information recorded. The laboratory will add such information as it needs during registration; however any changes to data originally entered by the officer should be audited and reported back to the relevant competent authorities client. Once registered, samples are treated in exactly the same way as any other sample within the laboratory. Only at sample approval is there any difference from other samples, see “Outcome Recording” below.
Once samples have been approved and reported, the data is exported from LIMS in a predefined format and sent electronically to the FSS web administrator. The new data is incorporated into the FSS database, overwriting existing data if appropriate. Finally, the competent authorities clients download their own data from the FSS web site thus updating their own systems.
It can be seen that:-
There is a training issue for the LIMS administrator
There is an ongoing maintenance issue for the LIMS administrator
There is a training issue for sample reception staff
There is a training issue for sample approvers
There is no impact on other staff who use LIMS
Any changes made to sample data as it passes through the laboratory LIMS are written back to the Client’s system thus updating their system.
1.1. System diagram
1.2. Data Structures
The data consists of a primary table of samples data along with associated results and labelling outcomes. The sample information is largely completed by the competent authority. The lab then applies a satisfactory/unsatisfactory judgement and comments along with a set of results and outcomes. The data structure and reference lists can be seen in appendices II – IX.
1.3. Outcome recording
In order to provide the Competent Authority client with sufficient information to complete their FSA returns, it is necessary to indicate the category and judgement of each test result. The result outcome category is related to the determination applied. Further, labelling failures should be recorded against the sample. (See appendix VIII)It is understood that the only person able to make this judgement is the person approving the sample; only they have all of the analysis information available and the knowledge or experience to decide the categories of examination and failure. Consequently, at sample approval the approver will be presented with a dialogue to gather the appropriate information. It should not be possible to skip this dialogue. As the great majority of results will be “satisfactory”, it is useful to set this value as the default for all tests. The approver need only change the outcome for any or all results deemed to be unsatisfactory. A validation check should be enabled which prevents a sample being recorded as satisfactory overall while having one or more unsatisfactory outcomes recorded against it. Similarly an unsatisfactory sample must have at least one unsatisfactory outcome.
Reference Lists
1.3.1. Lab determinations
In order to avoid the need for each participating laboratory to use the same set of determination codes (Dets), a standardised list of determinations has been created (FD_Dets). Please see an extract of this reference list in Appendix VII. This list gives a code to each analyte/units combination taking no notice of methodology (except in a very few cases). It is a task for the Laboratory’s LIMS administrator to match their internal laboratory Det with the corresponding FD_Det.
The precise method for doing this will depend on the LIMS application in use. This scheme should allow for the possibility of several Dets to have the same FD_Det. This process requires to be completed once only for each relevant Det. Not all Dets in any particular laboratory system will require an FD_Det entry as only reportable Dets related to FSS samples need be included.
The FSS Support Team maintains the FD_Dets list and any requests for additional FD_Dets require to be raised with them.
The FSS Support Team will provide the designated contact person within each of the participating laboratories with an updated FD-Dets table, when necessary, via e-mail. On receipt, the updated table should be used to overwrite the existing table. The accompanying e-mail text will, normally, specify the additions or changes and can be used to check if any are relevant to the recipient laboratory.
Laboratories are responsible for the integrity of the matching process. Assistance may be sought from The FSS Support Team in providing tools to assist in completing this process. The FSS Support Team will periodically audit the matches at a particular laboratory and will require any corrections to be carried out timeously.
Please note that only results which are included in the final report to the client should be exported to FSS. All other results, e.g. intermediate results, should be ignored.
1.3.2. Food/feed categorisation
This is a reference list that provides all the food and feed categories currently in use. This list is fairly stable and rarely needs to be updated. Any updates will be circulated by The FSS Support Team. See appendix IV. A guidance document on food categorisation is also available on the FSS website: https://www.envhealth.scot.nhs.uk/public/Documents/
1.3.3. Outcomes and Outcome Codes
Each test result will have an outcome code applied (see appendix VIII), for the great majority of results, this will be the ‘satisfactory’ code. Furthermore, labelling outcomes are recorded against the sample in a separate outcomes table (see appendix VIII).
The standard Outcome Code lists (in a suitable format) can be obtained from The FSS Support Team.
1.3.4. Translation tables
In many LIMS, it will be necessary to set up a process whereby the data supplied in the FSS data file is matched to appropriate locations (fields) in the system. Similarly data exported must be translated from the LIMS structure into that expected by FSS. Clearly the manner in which this is achieved is system dependent. The input and output data files use an xml schema (see Appendix IX).
1.4. Information tables
1.4.1. AUDITING
The LIMS should be capable of auditing changes made to the data supplied by FSS and periodically reporting these changes back to the relevant Competent Authority client.
1.4.2. OUTCOME
A process requires to be put in place to record the “Outcomes” (using the Outcome Codes as listed in appendix VIII) for each test applied to an FSS sample analysed at the laboratory. The Outcome data must be exported to FSS in an agreed structure.
1.5. Changes to existing LIMS tables
1.5.1. Additional Fields
It is highly likely that data relating to FSS samples will not be recorded currently in the LIMS in use. It will be necessary to be able to handle this additional data either by the creation of new, linked, tables or by the addition of fields to existing tables. The precise process to be used is system dependant but must maintain the integrity of the FSS data.
1.5.2. Changes to Data type and/or length
It might also be necessary to amend the data type or field length of existing data fields so as to accept the FSS data.
The disposition of the sample i.e. Satisfactory / Unsatisfactory needs to be recorded and transmitted to the FSS database. The structure for the FSS data includes this item.
1.6. Changes to LIMS processes
Apart from certain operations, e.g. sample registration and data export, there should be minimal impact on most LIMS processes. The main aim would be to remove any differentiation between FSS samples and others during the laboratory analysis and result recording process.
1.7. Changes to screens or additional screens
In order to accommodate FSS samples, some screens may need to be redesigned.
However this would be entirely system specific.
1.8. Other changes
1.8.1. Sample Reports
Sampling Officers name
The standard information entered into FSS for the sampling officer is “Name” plus “Tel Number” and/or “E-Mail”. For some reports, it is necessary to hide, or remove, the unwanted text and leave only the contact name. This can be done by editing the LIMS sample record but is better achieved by modifying the report itself as this retains the integrity of the source data. The following guidance applies to ReportPro reports but is directly translatable into other report engines including R&R. In the report field which prints the sampling officer’s name, change the expression to:
AllTrim( Left( Samples.Sampled_By, At(“-”, Samples.Sampled_By) -1)) Note:
This assumes that a dash (hyphen) has been used to separate the sampling officer’s name and the rest of the information in FSS.
Replace the field name “Samples.Sampled_By” with the appropriate one for your own system.
The “At()” function identifies the position of the “-“ in the sampled_by string. Hence the use of “-1” to exclude the hyphen itself from the name.
The leading “AllTrim” removes leading and trailing spaces from the generated string.
1.9. Routine Tasks
1.9.1. Audit reports to clients
As changes to sample details within LIMS are written back to the client’s FSS database, it is useful to highlight such changes to the clients. This serves two purposes; it is both a courtesy and serves as a training point.
Where changes made during sample registration are recorded, it is recommended that reports of such changes are sent to the relevant clients on a weekly basis for all FSS samples registered during the previous 7 days.
Where changes are made at later stages in the sample’s progress through the laboratory, it is recommended that extracts from this audit table be sent to the relevant clients on a monthly basis. This report should cover changes made to sample details during the previous calendar month.
1.10. Updating Reference tables
1.10.1. Maintain determinations
It is necessary to ensure that the local Dets list is kept up to date with relevant FD_Det entries. It will be necessary to provide evidence to HPS on an annual basis, that the mapping is accurate and up to date. Please contact the FSS Support Team in order to agree how this might be carried out.
1.10.2. Maintain the food & feed categorisation
It is essential that any internal reference lists which are used in sample categorisation are maintained up to date with the latest available reference lists. The reference lists are obtainable from the FSS Support Team.
1.11. Export FSS data
Data, including sample details, results, comments and outcomes, require to be exported from LIMS to the FSS website. This should be carried out regularly, not less than weekly. Only FSS samples which have been approved/reported and which have not previously been exported should be included in each export process.
Whenever data is exported, it must be encrypted and sent to the FSS web administrator. An agreed filename structure should be used. Details of this process are described elsewhere. It is helpful if a brief e-mail message giving the number of samples exported is sent to the FSS web administrator.
1.12. Re-export FSS data
In some circumstances, sample data may need to be re-exported. The export process should be capable of achieving this. In the event of intentional re-export, the web administrator of FSS should be informed of the number of samples involved.
1.13. Notes
1.13.1. Pre-registration of samples into LIMS
For a number of reasons, it is occasionally necessary to register samples into LIMS before the data file containing the FSS sample details has been received. The LIMS should be capable of achieving this without compromising the FSS data.
With the exception of the field listed below, the incoming data should overwrite any existing data in the LIMS sample record. Any such changes should be recorded in the audit trail for the sample with a standard reason. Any empty sample record fields can be updated where appropriate but there need not be an audit record in this case.
The field which it is recommended should NOT be changed during the input process is:-
Food category
In this way, the sample categorisation applied at the lab, along with the associated suite and work information, is never changed.
1.13.2. Outcomes - LIMS Outcome Recording
A routine for recording reasons for failing a sample and for identifying the category of the work carried out.
Food samples are routinely analysed for one or more of the following categories of test
o Bacteriology o Additive o Constituent o Nutritional Component o Undesirable Substances o Substitution o Labelling
In order to be able to complete FSA returns, both Public Analysts and
Environmental Health Officers require to know:- 1. Count of Samples analysed for each category
Count of Samples failed in each category
A detailed breakdown of failures in each category
It is recognised that a scheme for collecting outcome data must:
Avoid undue additional input from users
Be capable of extension as areas of work other than foods are incorporated
Be tailored in such a way that export of the data to the Food Surveillance System (FSS) is feasible
The reference list for outcomes data should be obtained from the FSS Support Team.
1.14. Multi-component Samples
“Multi-component” samples are those where a single sample is submitted for analysis, but which is more conveniently split into 2 or more component parts. Examples of this are 5-part “Formal” dairy samples (milks, creams etc) for microbiological examination or packs of sweets where the colour content of each colour of sweet is identified and quantified separately. The following process ensures that related sample data is linked together on the FSS database.
Register the sample in the normal way from the data file supplied by the client
Using this sample record as a “seed”, replicate the record sufficient to provide a sample record for each separate component of the sample. Ensure that the national reference number for the sample is replicated in each component record.
The sample description text may be edited so as to describe the component to which the record relates.
The samples should be processed in the laboratory and have satisfactory/unsatisfactory and outcome data recorded as usual.
All data for the sample and its components must be exported to FSS in the same batch.
2. Appendix I : Data Definitions
2.1. Sample Data
Sample Data |
|||||||
|---|---|---|---|---|---|---|---|
CFAD Field Name |
Data Type |
Data Length |
Allows NULLS? |
Default |
Validation Rules |
XML Field Name |
Description |
RECORD_TYPE |
varchar |
30 |
No |
FOOD’ |
RecordType |
Contains information on the type of record (FOOD or ANIMAL FEED). |
|
‘ANIMAL FEED’ |
|||||||
SAMPLENO |
varchar |
30 |
No |
SampleNumber |
Unique sample number allocated by the Data Capture Program for the sample. Combined with LOCALUTH and OFFICE provides a unique reference for the sample record. |
||
LOCALAUTH |
char |
3 |
No |
This references the |
LocalAuthorityCode |
Three-letter code from the Local Authorities table identifies the Local |
|
refLocalAuthorities table with a list of local authority codes. Please see appendix III Local Authorities |
Authority. The reference list is updated as new local authorities join the system. |
||||||
NA |
LocalAuthorityName |
Only within xml schema. For Local Authority interface. |
|||||
Full name of the Local |
|||||||
Authority |
|||||||
OFFICE |
varchar |
5 |
No |
(‘HQ’) |
LocalAuthorityOfficeCode |
Unique (up to three-letter) code identifying the local authority office. |
|
NA |
CombinedLACodeAndOfficeCode |
The combined Local |
|||||
Authority and Local |
|||||||
Authority Office code e.g. ‘876HQ’. This is required for sample registration in the AIS-LIMS. |
|||||||
NA |
LocalAuthorityOfficeName |
Only within xml schema. For Local Authority interface. Name of the Local Authority Office. |
|||||
LA_SAMPLENO |
varchar |
50 |
YES |
LocalAuthoritySamplenumber |
Unique sample number allocated by the Local Authority to identify the sample. Optional. |
||
LIMSNO |
varchar |
50 |
YES |
LIMSSampleNumber |
Optional Laboratory Information Management System (LIMS) reference. |
||
ANALYSISTYPE |
char |
1 |
No |
(‘M’) |
Single letter code |
AnalysisType |
Required code identifying the analysis type. |
‘M’ - micro sample |
|||||||
‘C’ - chemical sample |
|||||||
NA |
SamplingOfficerCode |
Only within XML schema. For Local Authority interface. Code for the sampling officer. Used to match with sampling officer code within Local Authority Management System. |
|||||
SAMPLOFFR |
varchar |
50 |
No |
SampleOfficerName |
This field identifies the sampling officer. Free format text (including null). Local users may wish to be able to modify this, but for valid data analysis, a consistent format is recommended. |
||
DATESTAMP |
datetime |
NULL |
No |
Date: dd/mm/yyyy |
DateSampleTaken TimeSampleTaken |
Identifies the date and time of the sample. Can be exported using two xml fields if system unable to export a datetime combined value. |
|
Time: (24hour format) hh:mm |
|||||||
PREMNAME |
varchar |
50 |
No |
PremisesName |
Free text name of the premises. |
||
BUSINESSID |
varchar |
25 |
YES |
BusinessId |
Optional. Up to 15-letter code identifying the business. Each Local Authority will use their own reference tables for this field. |
||
BUSADD1 |
varchar |
50 |
YES |
BusinessAddress1 |
Business Address, line 1 |
||
BUSADD2 |
varchar |
50 |
YES |
BusinessAddress2 |
Business Address, line 2 |
||
BUSADD3 |
varchar |
50 |
YES |
BusinessAddress3 |
Business Address, line 3 |
||
BUSADD4 |
varchar |
50 |
YES |
BusinessAddress4 |
Business Address, line 4 |
||
BUSPCODE |
varchar |
12 |
YES |
BusinessPostcode |
Business Post Code. |
||
PREMCODE |
char |
1 |
No |
A single letter code. References refPremisesTypes or refAFPremisesTypes ‘A’ - Primary Producers |
PremisesTypeCode |
Single character identifying the FSA category of premises. Mandatory. |
|
‘B’ - Slaughterhouses |
|||||||
‘C’ - Manufacturers/processors |
|||||||
‘D’ - Packers |
|||||||
‘E’ - Importers/Exporters |
|||||||
‘F’ - Distributors/Transporters |
|||||||
‘G’ - Retailers |
|||||||
‘H’ - Restaurants and other Caterers |
|||||||
‘I’ - Materials and Articles |
|||||||
Manufacturers and Suppliers |
|||||||
‘J’ - Manufacturers mainly selling by retail |
|||||||
‘K’ - Producers of feed materials |
|||||||
‘L’ - Stores of feed materials |
|||||||
‘M’ - Manufacturers of compound feed stuffs |
|||||||
‘N’ - Importers and representatives of establishments in third countries ‘O’ - Intermediaries, distributors and retailers |
|||||||
‘P’ - Manufacturers of additives and premixtures ‘Q’ - Animal farms |
|||||||
‘R’ - Other feed businesses |
|||||||
NA |
PremisesTypeDescription |
Only within XMl schema. For Local Authority interface. Description of the premises type. |
|||||
REASON |
char |
1 |
No |
A single letter code. References refReasons or refAFReasons ‘E’ - Enforcement/Investigation |
ReasonForTakingSampleCode |
Character field identifying the reason for the sample. Taken from a look-up table of possible reasons for the sample, plus “other”. |
|
‘S’ - Surveillance/Monitoring |
|||||||
NA |
ReasonForTakingSampleDescription |
Only within XML schema. For Local Authority interface. Description of Food Standards Risk Category. |
|||||
FOODSAMP |
char |
1 |
No |
A single letter code. References |
SampleTypeCode |
Character field identifying the type of the sample (Formal/Informal etc). |
|
refSAmpleTypes or refAFSampleTypes ‘C’ - Food Complaint |
Taken from a look-up table. |
||||||
‘F’ - Formal |
|||||||
‘I’ - Informal |
|||||||
‘M’ - Imported Food |
|||||||
‘Q’ - Control |
|||||||
NA |
SampleTypeDescription |
Only within XML schema. For Local Authority interface. Description of Sample Type. |
|||||
FOLLOWUP |
bit |
NULL |
YES |
IsFollowUpSample |
Yes/no field identifying if follow-up action is required. If set (yes), then the |
||
INDEXNO field requires an entry. |
|||||||
INDEXNO |
varchar |
30 |
YES |
FollowUpSampleReference |
Required if the FOLLOWUP field is set. |
||
RISKCAT |
char |
1 |
YES |
A single letter code in the range A |
FoodSafetyRiskCategoryCode |
Optional Risk category. |
|
|
Single character, no lookup. |
||||||
NA |
FoodSafetyRiskCategoryDescription |
Only within XML schema. |
|||||
For Local Authority |
|||||||
Interface. Description of |
|||||||
Food Safety Risk Category. |
|||||||
FSRISKCAT |
char |
1 |
YES |
A single letter code. |
FoodStandardsRiskCategoryCode |
Food Standards Rick |
|
‘A’ - High |
Category of Premise. Single |
||||||
‘B’ - Medium |
Character. Optional |
||||||
‘C’ - Low |
|||||||
NA |
FoodStandardsRiskCategoryDescription |
Only within XML schema. For Local Authority interfaceDescription of Food Standards Risk Category. |
|||||
FOODPOIS |
bit |
NULL |
YES |
IsFoodBourneIllnessInvestigation |
Yes/no field identifying if the sample is related to an incident of food poisoning. If set (yes), then the DETAILS field requires an entry. |
||
DETAILS |
varchar |
100 |
YES |
FoodBourneIllnessDetails |
Details of the food poisoning case indicated by FOODPOIS being set. |
||
SURVEY |
bit |
NULL |
YES |
IsSurvey |
Yes/no field identifying if the sample was taken as part of |
||
a survey. If set (yes), then the SURVEYBODY and |
|||||||
SURVEYNO fields require entries. |
|||||||
SURVEYBODY |
char |
1 |
YES |
A single letter code. References refSurveyBodies or refAFSurveyBodies |
SurveyBodyCode |
Character field identifying the survey body (participants). |
|
‘A’ - Local Authority |
|||||||
‘C’ - SFELC |
|||||||
‘D’ - Defra/SEERAD |
|||||||
‘E’ - EU |
|||||||
‘F’ - FSA |
|||||||
‘L’ - Local Liaison Group |
|||||||
‘O’ - LACORS |
|||||||
‘P’ - Public Analyst |
|||||||
‘R’ - DARD |
|||||||
‘X’ - Other |
|||||||
SURVEYNO |
varchar |
50 |
YES |
SurveyReference |
Required if the SURVEY field is set. |
||
BRANDNAME |
varchar |
100 |
YES |
BrandName |
Name of Brand. |
||
FOODDESCR |
varchar |
250 |
No |
FoodDescription |
Description of food sample. |
||
CATEGORY |
varchar |
2 |
No |
A code in the format ‘nn’. References refCatgeorisations - see Appendix IV – Food/Feed Categorisations |
CategoryCode |
1st level category code of refCATEGORISATIONS table entry for sample categorisation. |
|
NA |
CategoryDescription |
Only within XML Schema. For Local Authority interface. Description of the 1st level category. |
|||||
SUBCAT |
varchar |
11 |
No |
A code in the format ‘nn.nn’. References refCatgeorisations - see Appendix IV – Food/Feed Categorisations |
SubCategoryCode |
2nd level category code of refCATEGORISATIONS table entry for sample categorisation. |
|
NA |
SubCategoryDescription |
Only within XML Schema. For Local Authority interface. Description of the 2nd level category. |
|||||
SUBCAT2 |
varchar |
8 |
No |
A code in the format ‘nn.nn.nn’ References refCatgeorisations - see Appendix IV – Food/Feed Categorisations |
Subcategory2Code |
3rd level category code of refCATEGORISATIONS table entry for sample categorisation. |
|
NA |
Subcategory2Description |
Only within XML Schema. For Local Authority interface. Description of the 3rd level category. |
|||||
CATEGORISATION |
char |
11 |
No |
A code in the format ‘nn.nn.nn.nn’ References refCatgeorisations - see Appendix IV – Food/Feed Categorisations |
SubCategory3Code |
4th level category code of refCATEGORISATIONS table entry for sample categorisation. |
|
NA |
SubCategory3Description |
Only within XML Schema. For Local Authority interface. Description of the 4th level category. |
|||||
MAFFCODE |
char |
3 |
No |
A code in the range of M01 - M65. |
MAFFCategoryCode |
MAFF/FSA code of food sample. Each MAFF code corresponds to a 2nd level entry in the refCATEGORISATIONS table. |
|
NA |
MAFFCategoryDescription |
Only within XML Schema. For Local Authority interface. Description of the MAFF/FSA food category. |
|||||
ADDINFO |
varchar |
50 |
YES |
Micro samples only: |
AdditionalInformation |
For micro samples this contains additional information about where the sample was taken from: a producer or retailer. For chemical samples - this contains any additional information about the sample. |
|
‘P’ - Producer |
|||||||
‘R’ - Retailer |
|||||||
‘N’ - Not applicable |
|||||||
PRODUCT |
char |
1 |
YES |
A single letter code. References refProducts. ‘E’ - Ready to eat |
NatureOfProductCode |
A code for the nature of product e.g. ready to eat, processed etc. References the refPRODUCTS table. |
|
‘P’ - Processed |
|||||||
‘R’ - Raw |
|||||||
‘S’ - Swab |
|||||||
NA |
ProductDescription |
Only within XML Schema. For Local Authority interface. Description of the nature of product. |
|||||
MANUFACTURER |
varchar |
100 |
YES |
ManufacturerDescription |
Manufacturer name. |
||
DISTRIBUTOR |
varchar |
50 |
YES |
DistributerDescription |
Distributor name. |
||
IMPORTER |
varchar |
50 |
YES |
ImporterDescription |
Importer name. |
||
COUNTRY |
varchar |
25 |
YES |
CountryCode |
Country of origin. |
||
NA |
CountryDescription |
Only within XML Schema. For Local Authority interface. Description of the country of origin. |
|||||
PACKAGING |
char |
1 |
No |
A single letter code. References refPackaging or refAFPackaging. |
PackagingCode |
Character field identifying the type of packaging. Taken from a look-up table of packaging types. |
|
‘B’ - Not prepacked (bulk) ‘C’ - Prepacked for ultimate consumer |
|||||||
‘D’ - Prepacked for direct sale |
|||||||
‘N’ - Not prepacked |
|||||||
‘O’ - Not prepacked / date code information provided |
|||||||
‘P’ - Prepacked |
|||||||
NA |
PackagingDescription |
Only within XML Schema. For Local Authority interface. Description of the packaging type. |
|||||
NA |
memo |
PointOfSaleInfo |
This is the labelling information from nonprepacked foods. This is sent to the lab to allow an assessment of labelling to be recorded accurately. The information is not sent to the central database. |
||||
MATERIAL |
char |
1 |
YES |
A single letter code. References refMaterial. ‘B’ -Cardboard |
PackagingMaterialCode |
Packaging material of the food sample. Taken from a look-up table of material types. |
|
‘C’ - Can |
|||||||
‘G’ - Glass |
|||||||
‘O’ - Other ‘P’ - Plastic |
|||||||
NA |
PackagingMaterialDescription |
Only within XML Schema. For Local Authority interface. Description of the packaging material. |
|||||
OTHERMATERIAL |
varchar |
50 |
YES |
OtherPackagingMaterialDetails |
Description when “other” selected for MATERIAL. |
||
QUANTITY |
decimal |
NULL |
YES |
PackageQuantity |
Quantity of sample (to 2 |
||
d.p.). Used with PACKSIZE |
|||||||
PACKSIZE |
varchar |
20 |
YES |
GRAMS’ |
PackageUnitscode |
Sample pack size/units. |
|
‘KG’ |
|||||||
‘LITRES’ |
|||||||
‘ML’ |
|||||||
‘PINTS’ |
|||||||
NA |
PackageUnitsDescription |
Only within XML Schema. Description of the package units. |
|||||
BATCHNO |
varchar |
20 |
No |
PackageBatchNumber |
Batch number of sample. |
||
HEALTHMARK |
varchar |
30 |
YES |
PackageHealthmark |
Health mark of the food sample, if available. |
||
DURABILITY |
char |
1 |
No |
A single letter code. References refDurabilities or refAFDurabities. |
DurabilityCode |
Character field identifying the durability. |
|
‘B’ - Best before |
|||||||
‘E’ - Best before end |
|||||||
‘N’ - Not provided |
|||||||
‘U’ - Use by |
|||||||
‘X’ - Expiry date |
|||||||
‘I’ - Illegible |
|||||||
‘O’ – Other |
|||||||
NA |
DurabilityDescription |
Only within XML Schema. For Local Authority interface. Description of the durability. |
|||||
DURABDAY |
tinyint |
NULL |
YES |
Numbers in the range 1-31 |
DurabilityDay |
Durability day of month. |
|
DURABMTH |
tinyint |
NULL |
YES |
Numbers in the range 1 -12 |
DurabilityMonth |
Durability month (1 – 12). |
|
DURABYR |
smallint |
NULL |
YES |
Year in yyyy format. |
DurabilityYear |
Durability year |
|
CONDITION |
char |
1 |
No |
A single letter code. References refConditions or reAFConditions. |
ConditionCode |
Character field identifying the condition. |
|
‘A’ - Ambient |
|||||||
‘C’ - Chilled |
|||||||
‘F’ - Frozen |
|||||||
‘H’ - Hot |
|||||||
‘O’ - Other |
|||||||
NA |
ConditionDescription |
Only within XML Schema. For Local Authority interface. Description of the condition. |
|||||
OTHERCONDITION |
varchar |
50 |
YES |
OtherconditionDetails |
Further details of condition if other selected under CONDITION. |
||
TEMPERATURE |
decimal |
NULL |
YES |
Number to one decimal place. |
Temperature |
Temperature, deg C. |
|
COPCONDITION |
bit |
NULL |
No |
MeetsCodeOfPractice |
Yes/no field identifying if the sample was kept in accordance with codes of practice for condition. If no, then the NOTCOP field requires an entry. |
||
NOTCOP |
varchar |
50 |
YES |
CodeOfPracticeDetails |
Description of why the COPCONDITION flag is set to NO. |
||
COPLAB |
bit |
NULL |
YES |
MeetsCodeOfPracticeTransport |
Yes/no field identifying if the sample was taken and submitted in accordance with codes of practice for lab samples. |
||
LAB |
char |
5 |
No |
A five letter code for the testing laboratory. See appendix V – Laboratories. |
TestingLaboratoryCode |
5-character field identifying the lab. |
|
NA |
TestingLaboratoryDescription |
Only within XML Schema. For Local Authority interface. Description of the testing laboratory. |
|||||
SAMPCOMMENTS |
varchar |
1000 |
No |
SampleComments |
Free text field for additional remarks by EHO. |
||
NA |
varchar |
4000 |
No |
IsRoutineAnalysisRequired |
Only within XML Schema. |
||
Information for Laboratory. A true/false to indicate if routine analysis is requested. |
|||||||
LABCOMMENTS |
varchar |
4000 |
No |
LaboratoryComments |
The comments from the lab staff on the overall result of testing. |
||
PROSECUTION |
bit |
NULL |
YES |
Prosecution |
Not used at present. Signifies whether a sample subsequently led to prosecution. |
||
SATISFACTORY |
bit |
NULL |
No |
IsSatisfactory |
Used by the Lab to indicate if a sample was found to be unsatisfactory or satisfactory. |
||
LASATISFACTORY |
bit |
NULL |
YES |
LocalAuthSatisfactory |
Not used at present. Could be used by the Local Authority to indicate if they consider a sample to be satisfactory or unsatisfactory. |
||
FAILCODE |
char |
3 |
YES |
(Now redundant field – superseded by ‘FailCode2’ in Outcomes Data) |
FailCode |
Indicates how a sample has |
|
A failcode for micro samples. |
failed inspection. This information has now been incorporated into the ‘datOutcomes’ table. Can be used for labs which carry out micro analysis only. |
||||||
‘J’ - Acceptable |
|||||||
‘K’ - Unsatisfactory |
|||||||
‘L’ - Unacceptable |
|||||||
ANALYTICALINSTRUCTIONS |
varchar |
1000 |
No |
RoutineComments |
Free text field to allow the EHO to define specific analysis instructions for the sample. |
||
CFAD_INFO |
varchar |
500 |
No |
CFADInformation |
Contains the version number of the FSSNet application used to collect the sample data. |
||
REGISTRATION_NUMBER |
varchar |
15 |
YES |
AnimalFeedRegistrationNumber |
This is an Animal Feeds field and is the premises registration number |
||
DATE_OF_MANUFACTURE |
datetime |
NULL |
YES |
Date in format: dd/mm/yyyy |
AnimalFeedDateOfManufacture |
This is an Animal Feeds field and is the date of manufacture of the feeding stuff. |
|
ANIMAL_SPECIES_CODE |
varchar |
15 |
No |
Code in format nn.nn references refAFAnimalSpecies - see appendix VI – Animal Species. |
AnimalFeedSpeciesCode |
This is an Animal Feeds field and is the code for the animal species type of the feeding stuff. |
|
NA |
AnimalFeedSpeciesDescription |
Only within XML Schema. For Local Authority interface. Description of the animal species. |
|||||
LABEL_REGISTRATION_NUMBER |
varchar |
15 |
YES |
AnimalFeedLabelRegistrationNumber |
This is an Animal Feeds field and is the registration number from the animal feeding stuffs label. |
||
LABEL_BUSINESS_TYPE_CODE |
varchar |
15 |
YES |
Single letter code |
AnimalFeedLabelBusinessTypeCode |
This is an Animal Feeds field and is the code for the business type on the feed label. |
|
‘D’ -Distributor/Supplier |
|||||||
‘I’ - Intermediary |
|||||||
‘M’ - Manufacturer |
|||||||
‘N’ - Not specified |
|||||||
LABEL_BUSINESS_ADDRESS |
varchar |
1024 |
YES |
AnimalFeedLabelBusinessAddress |
This is an Animal Feeds field and is the business address from the feeding stuff label. |
||
REPORTED_DATE |
datetime |
NULL |
YES |
Date in format: dd/mm/yyyy |
RecordedDate |
The date when the results report was printed for despatch to the Local Authority. |
|
NA |
decimal |
PurchaseCost |
The cost of the sample to purchase. This is used for the interface with the Local Authority Management system and is not imported by the lab or sent to the central database. |
2.2. Result Table
Result Data |
|||||||
|---|---|---|---|---|---|---|---|
CFAD Field Name |
Data Type |
Data Length |
Allows NULLS? |
Default |
Validation Rules |
XML Field Name |
Description |
PK |
int |
NULL |
No |
N/A |
Primary key. Unique number generated by SQL Server |
||
SAMPLENO |
varchar |
30 |
No |
N/A |
Foreign key. Unique sample number associated with the sample. |
||
LOCALAUTH |
char |
3 |
No |
N/A |
Three-letter code from the Local Authorities table identifies the Local Authority. |
||
OFFICE |
char |
5 |
No |
N/A |
Unique (up to three-letter) code identifying the local authority office. |
||
RESULT |
float |
NULL |
YES |
Result |
Numerical result. |
||
TEXTRESULT |
varchar |
100 |
YES |
TextResult |
Textual results. |
||
RSLTTYPE |
bit |
NULL |
No |
Number code for type of result. |
ResultType |
Type of result. |
|
‘0’ - numerical result |
|||||||
‘1’ - textual result |
|||||||
QUALIFIER |
char |
1 |
YES |
Qualifier |
Result qualifier. |
||
DATESTAMP |
datetime |
NULL |
No |
Date: dd/mm/yyyy |
ResultTimeStamp |
Date and time of result. |
|
SAMPLETIME |
Time: (24hour format) hh:mm |
Used in Import table to record sample time if it cannot be exported within DATESTAMP. This is added to DATESTAMP during the import procedure. |
|||||
LAB |
char |
5 |
No |
A five letter code for the testing laboratory. |
N/A |
The Laboratory code. |
|
A five letter code for the testing laboratory. See appendix V – Laboratories. |
|||||||
DET |
varchar |
50 |
No |
A letter and number code representing the lab determination. |
Determination |
The lab determination code. Reference list maintained centrally and circulated by The FSS Support Team to all labs contributing to the system. |
|
References refLabDeterminations – For an example extract see appendix VII – Lab Determinations |
|||||||
OUTPUT |
varchar |
1000 |
YES |
Output |
Combined qualifier and result for reading. |
||
COMPONENT_DESC |
varchar |
100 |
YES |
ComponentDescription |
Used to identify parts of multi-component samples. Populated during import procedure. |
||
BATCH_NO |
varchar |
50 |
YES |
BatchNumber |
From LIMSNO in import table. Used for multicomponent samples. |
||
FOODDESCR |
varchar |
100 |
YES |
FoodDescription |
Taken from the FOODDESCR field in the samples table. Used when multi-samples imported. |
||
TEST_UNIT |
varchar |
100 |
YES |
TestUnits |
A field in the import table only. The test unit for the result. Used by non-AIS LIMS labs. This is used along with TEST_SUBSTAMCE during the import procedure to map to a standard DET. |
||
TEST_SUBSTANCE |
varchar |
100 |
YES |
TestSubstance |
A field in the import table only. The test substance for the result. Used by non-AIS LIMS labs. This is used during along with TEST_UNIT during the import procedure to map to a standard DET. |
||
FAILCODE |
varchar |
5 |
No |
A letter and number code representing the failcode. References refResultOutcomes. See appendix VIII Outcome Failcodes. |
FailCode |
This is the outcome failcode for the test applied. This captures the judgement on the result by the approver. |
|
N/A |
A description on the failure indicated by the FAILCODE. |
FailCodeDescription |
This is the description of the FAILCODE applied to this test. It can be used in the import process for cross-checking purposes, in the event of a data validation exception . |
2.3. Outcome Table
Outcome Data |
|||||||
|---|---|---|---|---|---|---|---|
CFAD Field Name |
Data Type |
Data Length |
Allows NULLS? |
Default |
Validation Rules |
XML Field Name |
Description |
SampleNo |
varchar |
30 |
No |
NULL |
NA |
Foreign key and Primary key along with FAILCODE2. Unique sample number associated with the sample. |
|
FailCode2 |
char |
3 |
No |
NULL |
A letter and number code representing the fail group. References refFailcodes2 - see appendix VIII Outcome Failcodes. |
Code |
Primary key along with SAMPLENO. The Outcome Code signifying the outcomes of the test results. |
NA |
Description |
Description of the outcomes failcode. |
4. Appendix III : Food/Feed Categorisations
4.1. Food Categorisations
4.2. Feed Categorisations
Code |
Parent Code |
Description |
|---|---|---|
50 |
Compound feeds |
|
50.01 |
50 |
Complete |
50.02 |
50.01 |
Complementary |
50.03 |
50.02 |
Feeds for particular nutritional purposes |
51 |
Feed materials |
|
51.01 |
51 |
Cereal grains, their products and by-products |
51.02 |
51.01 |
Oil seeds, oil fruits, their products and by-products |
51.03 |
51.02 |
Legume seeds, their products and by-products |
51.04 |
51.03 |
Tubers, roots, their products and by-products |
51.05 |
51.04 |
Other seeds and fruits, their products and by-products |
51.06 |
51.05 |
Forages and roughage |
51.07 |
51.06 |
Other plants, their products and by-products |
51.08 |
51.07 |
Milk products |
51.09 |
51.08 |
Land animal products |
51.1 |
51.09 |
Fish, other marine animals, their products and by-products |
51.11 |
51.1 |
Minerals |
51.12 |
51.11 |
Miscellaneous |
51.13 |
51.12 |
Certain protein sources (bioproteins) |
53 |
Premixtures |
|
53.01 |
53 |
Premixtures |
54 |
Drinking water |
|
54.01 |
54 |
Drinking water |
55 |
Feed Additives |
|
55.01 |
55 |
Preservatives |
55.02 |
55.01 |
Antioxidants |
55.03 |
55.02 |
Emulsifiers |
55.04 |
55.03 |
Stabilisers |
55.05 |
55.04 |
Thickeners |
55.06 |
55.05 |
Gelling Agents |
55.07 |
55.06 |
Binders |
55.08 |
55.07 |
Substances for control of radionucleide contamination |
55.09 |
55.08 |
Anti-caking agents |
55.1 |
55.09 |
Acidity Regulators |
55.11 |
55.1 |
Silage Additives |
55.12 |
55.11 |
Denaturants |
55.13 |
55.12 |
Colourants |
55.14 |
55.13 |
Flavouring Compounds |
55.15 |
55.14 |
Vitamins |
55.16 |
55.15 |
Compounds of Trace Elements |
55.17 |
55.16 |
Amino Acids, their salts and analogues |
55.18 |
55.17 |
Urea and its derivatives |
55.19 |
55.18 |
Digestibility Enhancers |
55.2 |
55.19 |
Gut Flora Stabilisers |
55.21 |
55.2 |
Substances which favourably affect the environment |
59 |
Not specified |
|
59.01 |
59 |
Not specified |
5. Appendix IV : Laboratories
Code |
Name |
|---|---|
AFBIF |
Agri-Food & Biosciences Inst, Newforge Food Chem |
AFBIH |
Agri-Food & Biosciences Institute, Hillsborough |
AFBIN |
Agri-Food & Biosciences Institute, Newforge GM |
AFBIS |
Agri-Food & Biosciences Inst, Newforge Salmonella |
AFBIV |
Agri-Food & Biosciences Institute, VSD Stoney Road |
NHABN |
NHS Lab, Aberdeen Royal |
NHASH |
HPA, Ashford |
NHBIR |
HPA, Birmingham |
NHBRI |
HPA, Bristol |
NHCHE |
HPA, Chelmsford |
NHCOL |
HPA, Colindale |
NHDG |
NHS Lab, Dumfries and Galloway |
NHLEE |
HPA, Leeds |
NHLEI |
HPA, Leicester |
NHPOR |
HPA, Porton Down |
NHPRE |
HPA, Preston |
NHSOU |
HPA, Southampton |
NHSTO |
HPA, Stoke-on-Trent |
NHTST |
HPA Starlims Test Laboratory |
NHYOR |
HPA, York |
PA-MW |
Minton Worcestershire Partnership |
PAABD |
Public Analyst Lab, Aberdeen |
PABIR |
Public Analyst Lab, Birkenhead (Eurofins) |
PABRI |
Public Analyst Lab, Bristol |
PACAR |
Public Analyst Lab, Cardiff |
PADUN |
Public Analyst Lab, Dundee |
PADUR |
Public Analyst Lab, Durham |
PAEDI |
Public Analyst Lab, Edinburgh |
PAGLA |
Public Analyst Lab, Glasgow |
PAHAM |
Public Analyst Lab, Hampshire |
PAKEN |
Public Analyst Lab, Kent |
PALAN |
Public Analyst Lab, Lancashire |
PALEI |
Public Analyst Lab, Leicester |
PALON |
Public Analyst Lab, London (Eurofins) |
PAMAN |
Public Analyst Lab, Manchester (Eurofins) |
PAMIN |
Public Analyst Lab, Minton |
PANIR |
Public Analyst Lab, Northern Ireland (Eurofins) |
PANOR |
Public Analyst Lab, Norwich |
PASOM |
Public Analyst Lab, Somerset |
PASTA |
Public Analyst Lab, Stafford |
PAWOL |
Public Analyst Lab, Wolverhampton (Eurofins) |
PAWOR |
Public Analyst Lab, Worcester |
PAWYO |
Public Analyst Lab, West Yorkshire |
PHBEL |
Public Health Lab, Belfast |
PHWBA |
Public Health Lab, Bangor |
PHWCD |
Public Health Lab, Cardiff |
PHWCM |
Public Health Lab, Carmarthen |
PHWRH |
Public Health Lab, Rhul |
LCOLB |
Labelling check only - no laboratory analysis |
PATST |
Test Laboratory |
PHWTE |
PHW Test Lab |
6. Appendix V : Animal Species
CODE |
ANIMAL TYPE CODE |
DESCRIPTION |
|---|---|---|
0.01 |
0 |
Cattle |
0.02 |
0 |
Cattle - Dairy cows |
0.03 |
0 |
Cattle - Beef cattle (breeding) |
0.04 |
0 |
Cattle for finishing |
0.05 |
0 |
Cattle - Calves for finishing |
0.06 |
0 |
Cattle - Calves |
0.07 |
0 |
Cattle - Ovines |
0.08 |
0 |
Sheep |
0.09 |
0 |
Sheep - Breeding sheep |
0.1 |
0 |
Sheep - Lambs for finishing |
0.11 |
0 |
Sheep - Lambs |
0.12 |
0 |
Goats |
0.13 |
0 |
Goats - Kids |
0.14 |
0 |
Bovines - other |
0.99 |
0 |
Bovines - not specified |
1.01 |
1 |
Porcines - Breeding pigs |
1.02 |
1 |
Porcines - Pigs for finishing |
1.03 |
1 |
Porcines - Pigs up to 16 weeks |
1.04 |
1 |
Porcines - Pigs 16 weeks to 6 months |
1.05 |
1 |
Porcines - Piglets |
1.06 |
1 |
Porcines - Other |
1.99 |
1 |
Porcines - Not specified |
2.01 |
2 |
Equines - Horses |
2.02 |
2 |
Equines - Ponies |
2.03 |
2 |
Equines - Foals |
2.04 |
2 |
Equines - other |
2.99 |
2 |
Equines - Not specified |
3.01 |
3 |
Rabbits - Breeding rabbits |
3.02 |
3 |
Rabbits - Rabbits for finishing |
3.03 |
3 |
Rabbits - Other |
3.99 |
3 |
Rabbits - Not specified |
4.01 |
4 |
Poultry - Turkey - fattening |
4.02 |
4 |
Poultry - Turkey - laying |
4.03 |
4 |
Poultry - Laying hens |
4.04 |
4 |
Poultry - Broiler chickens |
4.05 |
4 |
Poultry - Chicks |
4.06 |
4 |
Poultry - Ducks - laying |
4.07 |
4 |
Poultry - Ducks for fattening |
4.08 |
4 |
Poultry - Geese |
4.09 |
4 |
Poultry - Pigeons |
4.1 |
4 |
Poultry -Other |
4.99 |
4 |
Poultry - Not specified |
5.01 |
5 |
Fish - Salmon |
5.02 |
5 |
Fish - Trout |
5.03 |
5 |
Fish - Crustaceans |
5.04 |
5 |
Fish - Other |
5.99 |
5 |
Fish - Not specified |
6.01 |
6 |
OFPA - Alligators |
6.02 |
6 |
OFPA - Frogs |
6.03 |
6 |
OFPA - Kangaroos |
6.04 |
6 |
OFPA - Ostriches |
6.05 |
6 |
OFPA - Other |
6.99 |
6 |
OFPA - not specified |
7.01 |
7 |
Petfood - Cats |
7.02 |
7 |
Petfood - Dogs |
7.03 |
7 |
Petfood - Cage birds |
7.04 |
7 |
Petfood - Ornamental birds |
7.05 |
7 |
Petfood - Others |
7.99 |
7 |
Petfood - Not specified |
8.01 |
8 |
NFPA - Aquarium |
8.02 |
8 |
NFPA - Circus |
8.03 |
8 |
NFPA - Safari park |
8.04 |
8 |
NFPA - Zoo |
9.01 |
9 |
NFPA - Not specified |
7. Appendix VI : Lab Determinations (extract)
DET |
NAME |
UNITS |
Outcome Code 1 |
Outcome Text 1 |
Outcome Code 2 |
Outcome Text 2 |
Default |
|---|---|---|---|---|---|---|---|
Satisfactory |
|||||||
Outcome Code |
|||||||
CTER4OL-84 |
Terpinen-4-ol |
g/1000L alc |
B |
Constituent |
2 |
Alcohol |
0 |
CCAMPHO-84 |
Camphor |
g/1000L alc |
B |
Constituent |
2 |
Alcohol |
0 |
CLINALO-84 |
Linalool |
g/1000L alc |
B |
Constituent |
2 |
Alcohol |
0 |
CGERACE-84 |
Geranyl acetate |
g/1000L alc |
B |
Constituent |
2 |
Alcohol |
0 |
CAHUMUL-84 |
Alpha-humulene |
g/1000L alc |
B |
Constituent |
2 |
Alcohol |
0 |
CCASEINE |
Casein/Caseinates |
B |
Constituent |
5 |
Casein |
0 |
|
CCARBOH-81 |
Carbohydrate |
g/100 kcal |
B |
Constituent |
6 |
Carbohydrate |
0 |
CCARBPS-56 |
Carbohydrate per serving/meal |
g |
B |
Constituent |
6 |
Carbohydrate |
0 |
CCARBOH-86 |
Carbohydrate |
g/unit |
B |
Constituent |
6 |
Carbohydrate |
0 |
CTFAT-81 |
Fat - Total Fat |
g/100 kcal |
B |
Constituent |
8 |
Fat |
0 |
DFAT-44 |
Fat - declared |
g/100mL |
B |
Constituent |
8 |
Fat |
0 |
DFAT-86 |
Fat - declared |
g/unit |
B |
Constituent |
8 |
Fat |
0 |
D%NAMMEAT |
% of named meat - declared |
B |
Constituent |
14 |
Meat Content |
0 |
|
CPROTN-81 |
Protein |
g/100 kcal |
B |
Constituent |
17 |
Protein |
0 |
DPROTN-44 |
Protein - declared |
g/100mL |
B |
Constituent |
17 |
Protein |
0 |
DPROTN-86 |
Protein - declared |
g/unit |
B |
Constituent |
17 |
Protein |
0 |
BPHOS-30 |
Phosphatase |
ug/g |
B |
Constituent |
99 |
Other constituent |
0 |
BPHOS-04 |
Phosphatase |
ug/mL |
B |
Constituent |
99 |
Other constituent |
0 |
CPH1%SOL |
pH of 1% solution of Food Product |
B |
Constituent |
99 |
Other constituent |
0 |
|
CENERGY-66 |
Energy |
kcal/100mL |
C |
Nutritional Component |
2 |
Energy |
0 |
DENERGY-87 |
Energy Value - declared |
kcal/unit |
C |
Nutritional Component |
2 |
Energy |
0 |
DCARBOH-44 |
Carbohydrate - declared |
g/100mL |
C |
Nutritional Component |
6 |
Glucosamine |
0 |
DCARBOH-86 |
Carbohydrate - declared |
g/unit |
C |
Nutritional Component |
6 |
Glucosamine |
0 |
D-P-05 |
Phosphorus - declared |
mg/kg |
C |
Nutritional Component |
7 |
Non-metallic elements |
0 |
C-MG-10 |
Magnesium |
g/100g |
C |
Nutritional Component |
8 |
Nutrient metals |
0 |
D-MG-10 |
Magnesium - declared |
g/100g |
C |
Nutritional Component |
8 |
Nutrient metals |
0 |
D-CA-05 |
Calcium - declared |
mg/kg |
C |
Nutritional Component |
8 |
Nutrient metals |
0 |
D-MG-05 |
Magnesium - declared |
mg/kg |
C |
Nutritional Component |
8 |
Nutrient metals |
0 |
D-NA-05 |
Sodium - declared |
mg/kg |
C |
Nutritional Component |
8 |
Nutrient metals |
0 |
C-CA-79 |
Calcium |
mg/100 mL |
C |
Nutritional Component |
8 |
Nutrient metals |
0 |
C-CA-82 |
Calcium |
mg/100 kcal |
C |
Nutritional Component |
8 |
Nutrient metals |
0 |
C-FE-79 |
Iron |
mg/100 mL |
C |
Nutritional Component |
8 |
Nutrient metals |
0 |
C-FE-82 |
Iron |
mg/100 kcal |
C |
Nutritional Component |
8 |
Nutrient metals |
0 |
C-ZN-79 |
Zinc |
mg/100 mL |
C |
Nutritional Component |
8 |
Nutrient metals |
0 |
C-ZN-82 |
Zinc |
mg/100 kcal |
C |
Nutritional Component |
8 |
Nutrient metals |
0 |
C-NA-79 |
Sodium |
mg/100 mL |
C |
Nutritional Component |
8 |
Nutrient metals |
0 |
C-NA-82 |
Sodium |
mg/100 kcal |
C |
Nutritional Component |
8 |
Nutrient metals |
0 |
D-NA-86 |
Sodium - declared |
g/unit |
C |
Nutritional Component |
8 |
Nutrient metals |
0 |
D-OMEG3-06 |
Omega 3 long chain fatty acids - declared |
mg/100g |
C |
Nutritional Component |
9 |
Omega fatty acids |
0 |
D-OMEG3-10 |
Omega 3 long chain fatty acids - declared |
g/100g |
C |
Nutritional Component |
9 |
Omega fatty acids |
0 |
CVITA-80 |
Vitamin A |
mg/100mL |
C |
Nutritional Component |
11 |
Vitamins |
0 |
CVITA-83 |
Vitamin A |
ug/100 kcal |
C |
Nutritional Component |
11 |
Vitamins |
0 |
CVITE-80 |
Vitamin E |
mg/100mL |
C |
Nutritional Component |
11 |
Vitamins |
0 |
CVITE-82 |
Vitamin E |
mg/100 kcal |
C |
Nutritional Component |
11 |
Vitamins |
0 |
CE300-82 |
Vitamin C (Ascorbic Acid) |
mg/100 kcal |
C |
Nutritional Component |
11 |
Vitamins |
0 |
D-CO-05 |
Cobalt - declared |
mg/kg |
D |
Undesirable substances |
6 |
Heavy Metals |
0 |
D-CU-05 |
Copper - declared |
mg/kg |
D |
Undesirable substances |
6 |
Heavy Metals |
0 |
D-MN-05 |
Manganese - declared |
mg/kg |
D |
Undesirable substances |
6 |
Heavy Metals |
0 |
D-SE-05 |
Selenium - declared |
mg/kg |
D |
Undesirable substances |
6 |
Heavy Metals |
0 |
C-CD-71 |
Cadmium |
mg/sq dm |
D |
Undesirable substances |
6 |
Heavy Metals |
0 |
C-PB-71 |
Lead |
mg/sq dm |
D |
Undesirable substances |
6 |
Heavy Metals |
0 |
C-T2TX-01 |
T-2 Toxin |
ug/kg |
D |
Undesirable substances |
10 |
Mycotoxins |
0 |
C-HT2TX-01 |
HT-2 Toxin |
ug/kg |
D |
Undesirable substances |
10 |
Mycotoxins |
0 |
CETHOPR-05 |
Ethoprofos |
mg/kg |
D |
Undesirable substances |
13 |
Pesticide |
0 |
C-MELAM-05 |
Melamine |
mg/kg |
D |
Undesirable substances |
99 |
Other Undesirable Substances |
0 |
C-CLFR-08 |
Chlorine - Free residual |
mg/L |
D |
Undesirable substances |
99 |
Other Undesirable Substances |
0 |
C-CLTR-08 |
Chlorine - Total residual |
mg/L |
D |
Undesirable substances |
99 |
Other Undesirable Substances |
0 |
BAERO-19 |
Aeromonas Species |
cfu per Litre |
M |
Microbiology |
1 |
Aeromonas |
20 |
BCAMP-19 |
Campylobacter |
cfu per Litre |
M |
Microbiology |
3 |
Campylobacter |
20 |
BCPER-17 |
C.perfringens |
cfu per gram |
M |
Microbiology |
4 |
Clostridia |
20 |
BCPER-32 |
C.perfringens |
cfu per 50mL |
M |
Microbiology |
4 |
Clostridia |
20 |
BCOL-33 |
Coliform |
cfu per 250mL |
M |
Microbiology |
5 |
Coliforms |
20 |
BEC-28 |
E.coli |
cfu per mL |
M |
Microbiology |
7 |
E Coli |
20 |
BEC-29 |
E.coli |
cfu per 100mL |
M |
Microbiology |
7 |
E Coli |
20 |
BEC-33 |
E.coli |
cfu per 250mL |
M |
Microbiology |
7 |
E Coli |
20 |
BEC-31 |
E.coli |
cfu per unit |
M |
Microbiology |
7 |
E Coli |
20 |
BEC-17 |
E.coli |
cfu per gram |
M |
Microbiology |
7 |
E Coli |
20 |
BEC-18 |
E.coli |
cfu per 100g |
M |
Microbiology |
7 |
E Coli |
20 |
BENT-28 |
Enterobacteria |
cfu per mL |
M |
Microbiology |
9 |
Enterobacteriaceae |
20 |
BENT-17 |
Enterobacteriaceae |
cfu per gram |
M |
Microbiology |
9 |
Enterobacteriaceae |
20 |
BENT-31 |
Enterobacteria |
cfu per unit |
M |
Microbiology |
9 |
Enterobacteriaceae |
20 |
BFSTR-33 |
F.Strep |
cfu per 250mL |
M |
Microbiology |
10 |
Faecal Streptococci |
20 |
BFSTR-28 |
F.Strep |
cfu per mL |
M |
Microbiology |
10 |
Faecal Streptococci |
20 |
BFSTR-29 |
F.Strep |
cfu per 100mL |
M |
Microbiology |
10 |
Faecal Streptococci |
20 |
BLMON-17 |
Listeria mono |
cfu per gram |
M |
Microbiology |
14 |
Listeria |
20 |
BLISTER-27 |
Listeria Detection |
cfu per 25g |
M |
Microbiology |
14 |
Listeria |
20 |
BLMON-27 |
Listeria Mono Detection |
cfu per 25g |
M |
Microbiology |
14 |
Listeria |
20 |
BLMON-28 |
Listeria mono |
cfu per mL |
M |
Microbiology |
14 |
Listeria |
20 |
BAEMES-17 |
Aerobic mesophiles |
cfu per gram |
M |
Microbiology |
15 |
Mesophiles |
20 |
BANMES-17 |
Anaerobic mesophiles |
cfu per gram |
M |
Microbiology |
15 |
Mesophiles |
20 |
BPAER-33 |
PS. Aeruginosa |
cfu per 250mL |
M |
Microbiology |
17 |
Pseudomonas |
20 |
BPAER-29 |
PS. Aeruginosa |
cfu per 100mL |
M |
Microbiology |
17 |
Pseudomonas |
20 |
BSAL-27 |
Salmonella |
cfu per 25g |
M |
Microbiology |
18 |
Salmonella |
20 |
BSAL |
Salmonella |
M |
Microbiology |
18 |
Salmonella |
20 |
|
BSAL-19 |
Salmonella |
cfu per Litre |
M |
Microbiology |
18 |
Salmonella |
20 |
BSHIG-19 |
Shigella |
cfu per Litre |
M |
Microbiology |
19 |
Shigella |
20 |
BCSAU-28 |
S.aureus |
cfu per mL |
M |
Microbiology |
21 |
Staphylococci |
20 |
BSAUR-17 |
S.aureus |
cfu per gram |
M |
Microbiology |
21 |
Staphylococci |
20 |
BSAUR-31 |
S.aureus |
cfu per unit |
M |
Microbiology |
21 |
Staphylococci |
20 |
BSAUR-27 |
S.aureus |
cfu per 25g |
M |
Microbiology |
21 |
Staphylococci |
20 |
BSAURT |
S.aureus Toxin |
M |
Microbiology |
21 |
Staphylococci |
20 |
|
BANTHE-17 |
Anaerobic thermophiles |
cfu per gram |
M |
Microbiology |
22 |
Thermophiles |
20 |
BAETHE-17 |
Aerobic thermophiles |
cfu per gram |
M |
Microbiology |
22 |
Thermophiles |
20 |
BTC-29 |
Total Count |
cfu per 100mL |
M |
Microbiology |
24 |
TVC |
20 |
BTC-33 |
Total Count |
cfu per 250mL |
M |
Microbiology |
24 |
TVC |
20 |
BTC30-28 |
TVC30 |
cfu per mL |
M |
Microbiology |
24 |
TVC |
20 |
BMICRO-19 |
Microscopy |
cfu per Litre |
M |
Microbiology |
24 |
TVC |
20 |
BC37-37 |
TVC37 |
cfu per 5mL |
M |
Microbiology |
24 |
TVC |
20 |
BVIBRIO-27 |
Vibrio Detection |
cfu per 25g |
M |
Microbiology |
25 |
Vibrio |
20 |
BVIBRIO-19 |
Vibrio Detection |
cfu per Litre |
M |
Microbiology |
25 |
Vibrio |
20 |
BYM-29 |
Yeast & Mould |
cfu per 100mL |
M |
Microbiology |
26 |
Yeast and Mould |
20 |
BYM-17 |
Yeast & Mould |
cfu per gram |
M |
Microbiology |
26 |
Yeast and Mould |
20 |
BYM-28 |
Yeast & Mould |
cfu per mL |
M |
Microbiology |
26 |
Yeast and Mould |
20 |
BRTECAT |
PHLS RTE category |
M |
Microbiology |
99 |
Other Microbiology |
20 |
|
ZCOMMENTS |
Comments |
Z |
Miscellaneous |
1 |
Misc Sample Information |
0 |
|
ZAPPEARANC |
Appearance |
Z |
Miscellaneous |
1 |
Misc Sample Information |
0 |
|
ZODOUR |
Odour |
Z |
Miscellaneous |
1 |
Misc Sample Information |
0 |
|
ZSTARTDATE |
Date analysis started |
Z |
Miscellaneous |
1 |
Misc Sample Information |
0 |
|
ZSAMPREP |
Sample Preparation |
Z |
Miscellaneous |
1 |
Misc Sample Information |
0 |
|
ZTASTE |
Taste |
Z |
Miscellaneous |
1 |
Misc Sample Information |
0 |
8. Appendix VII : Outcome Failcodes
8.1. Food Complaint
Outcome Code 1 |
Outcome Text 1 |
Outcome Code 2 |
Outcome Text 2 |
|---|---|---|---|
S |
Contamination |
1 |
Foreign material - animal |
S |
Contamination |
2 |
Foreign material - general dirt |
S |
Contamination |
3 |
Foreign material - hair or fibre |
S |
Contamination |
4 |
Foreign material - filtration aid |
S |
Contamination |
5 |
Foreign material - glass |
S |
Contamination |
6 |
Foreign material - insect |
S |
Contamination |
7 |
Foreign material - medicinal material |
S |
Contamination |
8 |
Foreign material - metal |
S |
Contamination |
9 |
Foreign material - rock or mineral |
S |
Contamination |
10 |
Foreign material - micro-organisms, mould (not bacteria) |
S |
Contamination |
11 |
Foreign material - paper, cardboard |
S |
Contamination |
12 |
Foreign material - plastic, rubber |
S |
Contamination |
13 |
Foreign material - vegetable material |
S |
Contamination |
14 |
Foreign material - wood |
S |
Contamination |
99 |
Foreign material - other |
T |
Microbiology |
1 |
TVC |
T |
Microbiology |
2 |
Enterobacteriaceae |
T |
Microbiology |
3 |
Pathogenic organism |
U |
Quality |
1 |
Abnormal taste, odour |
U |
Quality |
2 |
Metallic taint |
U |
Quality |
3 |
Rancid |
U |
Quality |
4 |
Sour |
U |
Quality |
99 |
Other unsatisfactory quality |
Outcome Code 3 |
Outcome Text 3 |
|---|---|
60 |
Satisfactory |
61 |
Present |
62 |
Absent |
63 |
Exceeds numerical standard |
64 |
Below numerical standard |
8.2. Food Chemistry
Outcome Code 1 |
Outcome Text 1 |
Outcome Code 2 |
Outcome Text 2 |
|---|---|---|---|
A |
Additive |
1 |
Antioxidant |
A |
Additive |
2 |
Colouring matter |
A |
Additive |
3 |
Flavour enhancer |
A |
Additive |
4 |
Preservative |
A |
Additive |
5 |
Solvent |
A |
Additive |
6 |
Sweetener |
A |
Additive |
99 |
Other additive |
B |
Constituent |
1 |
Acidity |
B |
Constituent |
2 |
Alcohol |
B |
Constituent |
3 |
Alkaloids |
B |
Constituent |
4 |
Ash |
B |
Constituent |
5 |
Casein |
B |
Constituent |
6 |
Carbohydrate |
B |
Constituent |
7 |
Egg |
B |
Constituent |
8 |
Fat |
B |
Constituent |
9 |
Fatty Acids |
B |
Constituent |
10 |
Fish |
B |
Constituent |
11 |
Fruit |
B |
Constituent |
12 |
Gluten |
B |
Constituent |
13 |
Honey |
B |
Constituent |
14 |
Meat Content |
B |
Constituent |
15 |
Milk |
B |
Constituent |
16 |
Moisture |
B |
Constituent |
17 |
Protein |
B |
Constituent |
18 |
Sterols |
B |
Constituent |
19 |
Sugar |
B |
Constituent |
20 |
Water |
B |
Constituent |
99 |
Other constituent |
C |
Nutritional Component |
1 |
Chondroitin |
C |
Nutritional Component |
2 |
Energy |
C |
Nutritional Component |
3 |
Fatty Acids |
C |
Nutritional Component |
4 |
Fibre |
C |
Nutritional Component |
5 |
Follate |
C |
Nutritional Component |
6 |
Glucosamine |
C |
Nutritional Component |
7 |
Non-metallic elements |
C |
Nutritional Component |
8 |
Nutrient metals |
C |
Nutritional Component |
9 |
Omega fatty acids |
C |
Nutritional Component |
10 |
Sugars |
C |
Nutritional Component |
11 |
Vitamins |
C |
Nutritional Component |
99 |
Other nutritional components |
D |
Undesirable substances |
1 |
3-MCPD |
D |
Undesirable substances |
2 |
Antibiotic |
D |
Undesirable substances |
3 |
Food contact materials |
D |
Undesirable substances |
4 |
Fungicide |
D |
Undesirable substances |
5 |
GMO |
D |
Undesirable substances |
6 |
Heavy Metals |
D |
Undesirable substances |
7 |
Herbicide |
D |
Undesirable substances |
8 |
Histamine |
D |
Undesirable substances |
9 |
Insecticide |
D |
Undesirable substances |
10 |
Mycotoxins |
D |
Undesirable substances |
11 |
Non-Permitted colour |
D |
Undesirable substances |
12 |
PCB |
D |
Undesirable substances |
13 |
Pesticide |
D |
Undesirable substances |
14 |
THM |
D |
Undesirable substances |
99 |
Other Undesirable Substances |
E |
Substitution |
1 |
Fish Identification |
E |
Substitution |
2 |
Meat Identification |
E |
Substitution |
3 |
Plant Identification |
E |
Substitution |
99 |
Other substitution |
U |
Quality |
1 |
Abnormal taste, odour |
U |
Quality |
2 |
Metallic taint |
U |
Quality |
3 |
Rancid |
U |
Quality |
4 |
Sour |
U |
Quality |
99 |
Other unsatisfactory quality |
X |
Radioactivity |
1 |
Radioactivity monitoring |
X |
Radioactivity |
2 |
Irradiated foods |
Z |
Miscellaneous |
1 |
Misc Sample Information |
Z |
Miscellaneous |
2 |
Labelling |
Outcome Code 3 |
Outcome Text 3 |
|---|---|
0 |
Satisfactory |
1 |
Above Limit/Declaration/Guideline |
2 |
Below Limit/Declaration/Guideline |
3 |
Present Not Permitted |
4 |
Present |
5 |
Absent |
8.3. Food Microbiology
Outcome Code 1 |
Outcome Text 1 |
Outcome Code 2 |
Outcome Text 2 |
|---|---|---|---|
M |
Microbiology |
1 |
Aeromonas |
M |
Microbiology |
2 |
Bacillus |
M |
Microbiology |
3 |
Campylobacter |
M |
Microbiology |
4 |
Clostridia |
M |
Microbiology |
5 |
Coliforms |
M |
Microbiology |
6 |
Cryptosporidia |
M |
Microbiology |
7 |
E Coli |
M |
Microbiology |
8 |
E Coli 0157 |
M |
Microbiology |
9 |
Enterobacteriaceae |
M |
Microbiology |
10 |
Faecal Streptococci |
M |
Microbiology |
11 |
Giardia |
M |
Microbiology |
12 |
Lactobacillus |
M |
Microbiology |
13 |
Legionella |
M |
Microbiology |
14 |
Listeria |
M |
Microbiology |
15 |
Mesophiles |
M |
Microbiology |
16 |
Micrococci |
M |
Microbiology |
17 |
Pseudomonas |
M |
Microbiology |
18 |
Salmonella |
M |
Microbiology |
19 |
Shigella |
M |
Microbiology |
20 |
Somatic Cell Count |
M |
Microbiology |
21 |
Staphylococci |
M |
Microbiology |
22 |
Thermophiles |
M |
Microbiology |
23 |
Thermoduric Bacteria |
M |
Microbiology |
24 |
TVC |
M |
Microbiology |
25 |
Vibrio |
M |
Microbiology |
26 |
Yeast and Mould |
M |
Microbiology |
27 |
Yersinia |
M |
Microbiology |
99 |
Other Microbiology |
Outcome Code 3 |
Outcome Text 3 |
|---|---|
0 |
Satisfactory |
1 |
Above Limit/Declaration/Guideline |
2 |
Below Limit/Declaration/Guideline |
3 |
Present Not Permitted |
4 |
Present |
5 |
Absent |
8.4. Animal Feeds
Outcome Code 1 |
Outcome Text 1 |
Outcome Code 2 |
Outcome Text 2 |
|---|---|---|---|
P |
Additives |
1 |
Acidity Regulators |
P |
Additives |
2 |
Antibiotics |
P |
Additives |
3 |
Antioxidant substances |
P |
Additives |
4 |
Binders, anti-caking agents, coagulants |
P |
Additives |
5 |
Bioproteins (unapproved) |
P |
Additives |
6 |
Coccidiostats and other medicinal substances |
P |
Additives |
7 |
Colourants, including pigments |
P |
Additives |
8 |
Emulsifying, stabilising, thickening, gelling substances |
P |
Additives |
9 |
Enzymes |
P |
Additives |
10 |
Flavouring, appetising substances |
P |
Additives |
11 |
Growth promoters |
P |
Additives |
12 |
Micro-organisms |
P |
Additives |
13 |
Preservatives |
P |
Additives |
14 |
Radionuclide binders |
P |
Additives |
15 |
Trace elements |
P |
Additives |
16 |
Vitamins, pro-vitamins, substances with similar effect |
P |
Additives |
99 |
Other additives |
Q |
Composition |
1 |
Protein, Nitrogen |
Q |
Composition |
2 |
Amino acids |
Q |
Composition |
3 |
Metals |
Q |
Composition |
4 |
Sugars, starch |
Q |
Composition |
5 |
Other elements and compounds |
Q |
Composition |
6 |
Moisture, Ash |
Q |
Composition |
7 |
Fibre |
Q |
Composition |
8 |
Oils, Fats |
Q |
Composition |
9 |
Sodium, salt |
Q |
Composition |
10 |
GMO |
Q |
Composition |
11 |
Labelling |
Q |
Composition |
99 |
Other constituents |
R |
Undesirable Substances |
1 |
Heavy metals |
R |
Undesirable Substances |
2 |
Other elements and ions |
R |
Undesirable Substances |
3 |
Dioxins, PCBs |
R |
Undesirable Substances |
4 |
Pesticides |
R |
Undesirable Substances |
5 |
Mycotoxins |
R |
Undesirable Substances |
6 |
Micro-organisms |
R |
Undesirable Substances |
7 |
Seeds, Fruits |
R |
Undesirable Substances |
8 |
Processed animal protein |
R |
Undesirable Substances |
99 |
Other undesirable substances |
Outcome |
Outcome Text 3 |
|---|---|
Code 3 |
|
40 |
Satisfactory |
41 |
Above maximum permitted level or standard |
42 |
Below maximum permitted level or standard |
43 |
Above action threshold |
44 |
Below action threshold |
45 |
Above recommended level |
46 |
Below recommended level |
47 |
Present |
48 |
Absent |
8.5. Labelling
Outcome Code 1 |
Outcome Text 1 |
Outcome Code 2 |
Outcome Text 2 |
|---|---|---|---|
L |
Labelling |
1 |
All statutory information |
L |
Labelling |
2 |
Name of the food |
L |
Labelling |
3 |
Name and/or address of manufacturer |
L |
Labelling |
4 |
Ingredients, Ingredient list |
L |
Labelling |
5 |
Durability indication |
L |
Labelling |
6 |
Place of origin |
L |
Labelling |
7 |
Storage and/or usage instructions |
L |
Labelling |
8 |
QUID declaration |
L |
Labelling |
9 |
Allergen declaration |
L |
Labelling |
10 |
Nutritional declaration |
L |
Labelling |
11 |
Other statutory or compulsory declaration |
L |
Labelling |
12 |
Medicinal or Health claim |
L |
Labelling |
13 |
Manufacture date, Expiry date |
L |
Labelling |
14 |
Net quantity |
L |
Labelling |
15 |
Wholly or partly illegible |
Outcome Code 1 |
Outcome Text 1 |
Outcome Code 2 |
Outcome Text 2 |
|---|---|---|---|
S |
Contamination |
1 |
Foreign material - animal |
S |
Contamination |
2 |
Foreign material - general dirt |
S |
Contamination |
3 |
Foreign material - hair or fibre |
S |
Contamination |
4 |
Foreign material - filtration aid |
S |
Contamination |
5 |
Foreign material - glass |
S |
Contamination |
6 |
Foreign material - insect |
S |
Contamination |
7 |
Foreign material - medicinal material |
S |
Contamination |
8 |
Foreign material - metal |
S |
Contamination |
9 |
Foreign material - rock or mineral |
S |
Contamination |
10 |
Foreign material - micro-organisms, mould (not bacteria) |
S |
Contamination |
11 |
Foreign material - paper, cardboard |
S |
Contamination |
12 |
Foreign material - plastic, rubber |
S |
Contamination |
13 |
Foreign material - vegetable material |
S |
Contamination |
14 |
Foreign material - wood |
S |
Contamination |
99 |
Foreign material - other |
T |
Microbiology |
1 |
TVC |
T |
Microbiology |
2 |
Enterobacteriaceae |
T |
Microbiology |
3 |
Pathogenic organism |
U |
Quality |
1 |
Abnormal taste, odour |
U |
Quality |
2 |
Metallic taint |
U |
Quality |
3 |
Rancid |
U |
Quality |
4 |
Sour |
U |
Quality |
99 |
Other unsatisfactory quality |
9. Appendix VIII : XML Schema
:linenos:
<?xml version="1.0" encoding="utf-8"?>
<xs:schema targetNamespace="" elementFormDefault="qualified" xmlns="http://www.maclarenwest.co.uk/fss/schema/FSSSample.xsd" xmlns:mstns="http://www.maclarenwest.co.uk/fss/schema/FSSSample.xsd" xmlns:xs="http://www.w3.org/2001/XMLSchema">
<xs:complexType name="CFADData">
<xs:sequence>
<xs:element name="FileInfo">
<xs:complexType>
<xs:sequence>
<xs:element name="DateGenerated" type="xs:string" />
<xs:element name="FileSource" type="xs:string" /> <xs:element name="SampleCount" type="xs:int" />
<xs:element name="SchemaVersion" type="xs:string" />
</xs:sequence>
</xs:complexType>
</xs:element>
<xs:element name="Sample" type="SampleType" maxOccurs="unbounded" />
</xs:sequence>
</xs:complexType>
<xs:complexType name="SampleType">
<xs:sequence>
<xs:element name="RecordType" type="xs:string" />
<xs:element name="SampleNumber" type="xs:string" />
<xs:element name="LocalAuthorityCode" type="xs:string" />
<xs:element name="LocalAuthorityName" type="xs:string" />
<xs:element name="LocalAuthorityOfficeCode" type="xs:string" />
<xs:element name="CombinedLACodeAndOfficeCode" type="xs:string" />
<xs:element name="LocalAuthorityOfficeName" type="xs:string" />
<xs:element name="LocalAuthoritySampleNumber" type="xs:string" />
<xs:element name="LIMSSampleNumber" type="xs:string" />
<xs:element name="AnalysisType" type="xs:string" />
<xs:element name="SamplingOfficerCode" type="xs:string" />
<xs:element name="SamplingOfficerName" type="xs:string" />
<xs:element name="DateSampleTaken" type="xs:date" />
<xs:element name="TimeSampleTaken" type="xs:time" />
<xs:element name="BusinessId" type="xs:string" />
<xs:element name="PremisesName" type="xs:string" />
<xs:element name="BusinessAddress1" type="xs:string" />
<xs:element name="BusinessAddress2" type="xs:string" />
<xs:element name="BusinessAddress3" type="xs:string" />
<xs:element name="BusinessAddress4" type="xs:string" /> <xs:element name="BusinessPostcode" type="xs:string" />
<xs:element name="PremisesTypeCode" type="xs:string" />
<xs:element name="PremisesTypeDescription" type="xs:string" />
<xs:element name="FoodSafetyRiskCategoryCode" type="xs:string" />
<xs:element name="FoodSafetyRiskCategoryDescription" type="xs:string" />
<xs:element name="FoodStandardsRiskCategoryCode" type="xs:string" />
<xs:element name="FoodStandardsRiskCategoryDescription" type="xs:string" />
<xs:element name="ReasonForTakingSampleCode" type="xs:string" />
<xs:element name="ReasonForTakingSampleDescription" type="xs:string" />
<xs:element name="SampleTypeCode" type="xs:string" />
<xs:element name="SampleTypeDescription" type="xs:string" /> <xs:element name="IsFollowupSample" type="xs:boolean" />
<xs:element name="FollowUpSampleReference" type="xs:string" />
<xs:element name="IsFoodBourneIllnessInvestigation" type="xs:boolean" />
<xs:element name="FoodBourneIllnessDetails" type="xs:string" />
<xs:element name="IsSurvey" type="xs:boolean" />
<xs:element name="SurveyBodyCode" type="xs:string" />
<xs:element name="SurveyBodyDescription" type="xs:string" />
<xs:element name="SurveyReference" type="xs:string" />
<xs:element name="BrandName" type="xs:string" />
<xs:element name="FoodDescription" type="xs:string" />
<xs:element name="CategoryCode" type="xs:string" />
<xs:element name="CategoryDescription" type="xs:string" />
<xs:element name="SubcategoryCode" type="xs:string" />
<xs:element name="SubcategoryDescription" type="xs:string" />
<xs:element name="Subcategory2Code" type="xs:string" />
<xs:element name="Subcategory2Description" type="xs:string" /> <xs:element name="Subcategory3Code" type="xs:string" />
<xs:element name="Subcategory3Description" type="xs:string" />
<xs:element name="MAFFCategoryCode" type="xs:string" />
<xs:element name="MAFFCategoryDescription" type="xs:string" />
<xs:element name="AdditionalInformation" type="xs:string" />
<xs:element name="NatureOfProductCode" type="xs:string" />
<xs:element name="ProductDescription" type="xs:string" />
<xs:element name="ManufacturerDescription" type="xs:string" />
<xs:element name="DistributerDescription" type="xs:string" />
<xs:element name="ImporterDescription" type="xs:string" />
<xs:element name="CountryCode" type="xs:string" />
<xs:element name="CountryDescription" type="xs:string" />
<xs:element name="PackagingCode" type="xs:string" />
<xs:element name="PackagingDescription" type="xs:string" />
<xs:element name="PointOfSaleInfo" type="xs:string" />
<xs:element name="PackagingMaterialCode" type="xs:string" />
<xs:element name="PackagingMaterialDescription" type="xs:string" />
<xs:element name="OtherPackagingMaterialDetails" type="xs:string" />
<xs:element name="PackageQuantity" type="xs:decimal" />
<xs:element name="PackageUnitsCode" type="xs:string" />
<xs:element name="PackageUnitsDescription" type="xs:string" />
<xs:element name="PackageBatchNumber" type="xs:string" />
<xs:element name="PackageHealthmark" type="xs:string" />
<xs:element name="DurabilityCode" type="xs:string" />
<xs:element name="DurabilityDescription" type="xs:string" />
<xs:element name="DurabilityDay" type="xs:int" />
<xs:element name="DurabilityMonth" type="xs:int" />
<xs:element name="DurabilityYear" type="xs:int" />
<xs:element name="ConditionCode" type="xs:string" />
<xs:element name="ConditionDescription" type="xs:string" />
<xs:element name="OtherConditionDetails" type="xs:string" />
<xs:element name="Temperature" type="xs:int" />
<xs:element name="MeetsCodeOfPractise" type="xs:boolean" />
<xs:element name="CodeOfPractiseDetails" type="xs:string" />
<xs:element name="MeetsCodeOfPractiseTransport" type="xs:boolean" />
<xs:element name="TestingLaboratoryCode" type="xs:string" />
<xs:element name="TestingLaboratoryDescription" type="xs:string" />
<xs:element name="SampleComments" type="xs:string" />
<xs:element name="IsRoutineAnalysisRequired" type="xs:boolean" />
<xs:element name="RoutineComments" type="xs:string" />
<xs:element name="LaboratoryComments" type="xs:string" />
<xs:element name="IsSatisfactory" type="xs:boolean" />
<xs:element name="FailCode" type="xs:string" />
<xs:element name="AnimalFeedRegistrationNumber" type="xs:string" />
<xs:element name="AnimalFeedDateOfManufacture" type="xs:string" /> <xs:element name="AnimalFeedSpeciesCode" type="xs:string" />
<xs:element name="AnimalFeedSpeciesDescription" type="xs:string" />
<xs:element name="AnimalFeedLabelRegistrationNumber" type="xs:string" />
<xs:element name="AnimalFeedLabelBusinessTypeCode" type="xs:string" />
<xs:element name="AnimalFeedLabelBusinessAddress" type="xs:string" />
<xs:element name="CFADInformation" type="xs:string" />
<xs:element name="RecordedDate" type="xs:date" />
<xs:element name="Prosecution" type="xs:boolean" />
<xs:element name="LocalAuthSatisfactory" type="xs:boolean" />
<xs:element name="PurchaseCost" type="xs:decimal" />
<xs:element name="Result" type="ResultType" maxOccurs="unbounded" />
<xs:element name="Outcome" type="OutcomeType" maxOccurs="unbounded" />
</xs:sequence>
</xs:complexType>
<xs:complexType name="ResultType">
<xs:sequence>
<xs:element name="Result" type="xs:decimal" />
<xs:element name="TextResult" type="xs:string" />
<xs:element name="ResultType" type="xs:string" />
<xs:element name="Qualifier" type="xs:string" />
<xs:element name="ResultTimeStamp" type="xs:dateTime" />
<xs:element name="Determination" type="xs:string" />
<xs:element name="TestSubstance" type="xs:string" />
<xs:element name="TestUnits" type="xs:string" />
<xs:element name="Output" type="xs:string" />
<xs:element name="ComponentDescription" type="xs:string" />
<xs:element name="BatchNumber" type="xs:string" />
<xs:element name="FoodDescription" type="xs:string" />
<xs:element name="FailCode" type="xs:string" />
<xs:element name="FailCodeDescription" type="xs:string" />
</xs:sequence>
</xs:complexType>
<xs:complexType name="OutcomeType">
<xs:sequence>
<xs:element name="Code" type="xs:string" />
<xs:element name="Description" type="xs:string" />
</xs:sequence>
</xs:complexType>
</xs:schema>
10. Appendix IX : EncExtract Setup
10.1. Setup
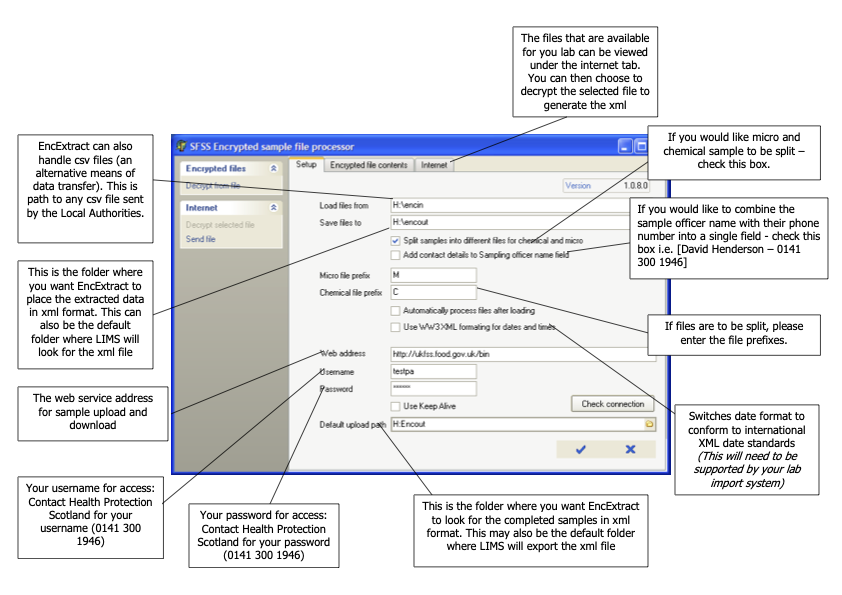
10.2. File download